The Clinical Valley of Death: Why Open Source Drug Development Struggles with Trial Funding
One of the most significant hurdles in open source drug development relies in clinical trials funding. Here's the core issue: When a project is fully open source, pharmaceutical companies become hesitant to invest in expensive clinical trials because they can't secure exclusive intellectual property rights. Clinical trials can cost hundreds of millions of dollars, and without the promise of market exclusivity to recoup these investments, traditional pharmaceutical companies have little financial incentive to fund them.
The COVID Moonshot example
The COVID Moonshot project is a prime example of the challenges faced by open source drug development. The project aimed to develop a vaccine for COVID-19 in record time and put all the project’s discovery scientific data in the public domain, but the project faced difficulties in securing funding to finance the clinical trials due to the lack of patent protection1. This highlights a significant challenge in open source research: traditional funding models often rely on the potential for future profits through intellectual property rights to justify investments in clinical trials. So projects like the COVID Moonshot that commit to fully open source drug development face a significant challenge in securing funding for clinical trials.
The COVID Moonshot team stated:2"During the COVID Moonshot project, we adopted a patent-free, open science direct-to-generic approach. This was highly effective in the research phase, enabling rapid recruitment of collaborators and remarkably fast transfer of ideas and data unencumbered by lengthy contract negotiations. However, it had several unforeseen consequences. Discussions with potential manufacturers were made more complex, as we could not guarantee that other parties would not develop production routes to the new antiviral in parallel, risking the recovery of their investment costs."
The ASAP Approach
In order to face these challenges the ASAP consortium (an AI-driven antiviral discovery platform that builds upon the COVID Moonshot project and expands its scope to target multiple viral families including coronaviruses, flaviviruses, and picornaviruses) created a hybrid model, combining open science with what they call a "minimally defensive patent" strategy. This approach aims to balance rapid development with the need for some IP protection to ensure equitable access. Their approach is described here: https://zenodo.org/records/12191567.
With ASAP hybrid model early research data is public, but promising candidates are temporarily protected through narrow patents before clinical trials. This compromise arose because manufacturers and funders required some IP protection to invest in development, while patents provided leverage for ensuring global access. Though partially open-source, this approach still required withholding some data and selective patenting to make development commercially viable.
Their strategy works as follows:
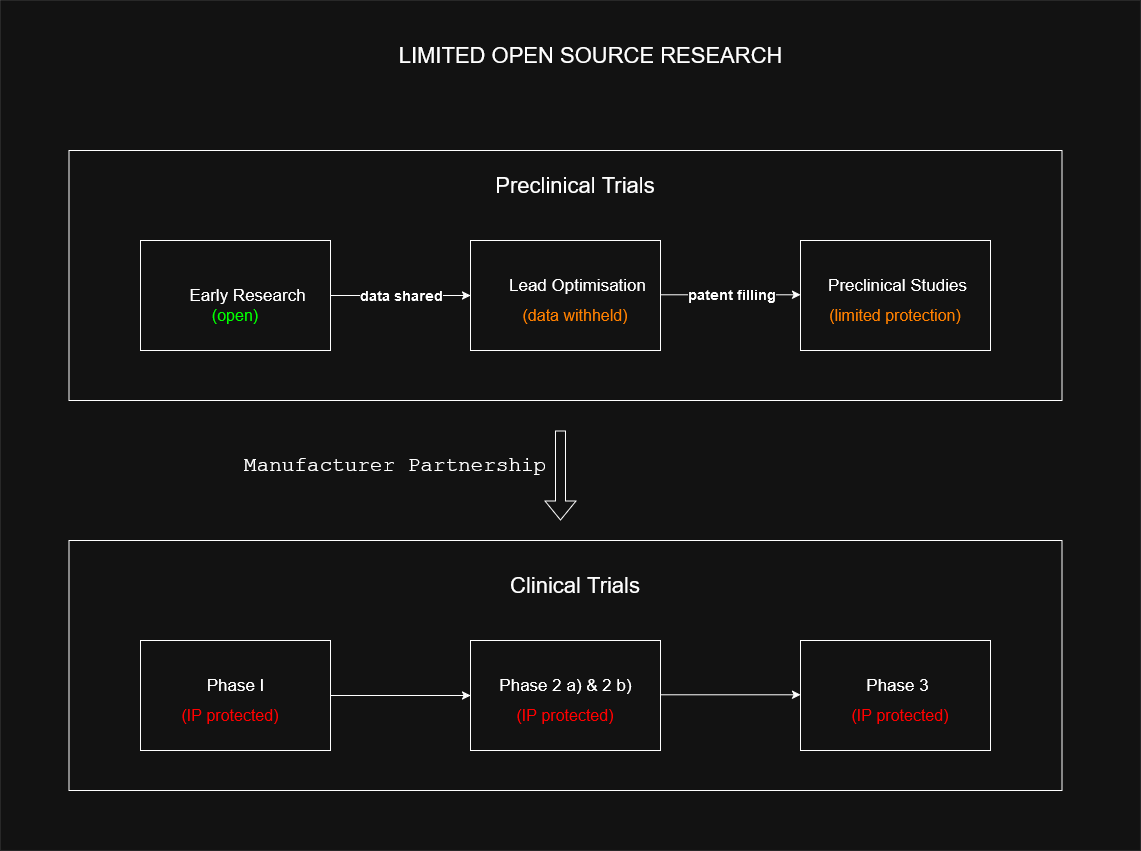
The ASAP consortium's approach still falls short of true open science principles. The need to withhold data during critical development phases and file patents (even if minimal) demonstrates how current pharmaceutical development frameworks remain fundamentally at odds with full open science practices. This highlights the need for new models that can better align the goals of open collaboration with the practical requirements of drug development and commercialization.
The Etica Protocol's Solution
The Etica Protocol offers a novel approach to open-source medical research and clinical trials that could revolutionize the drug development process, addressing many of the challenges faced by initiatives like the COVID Moonshot and ASAP Consortium.
In this article, we will explore:
- How Etica can fund open-sourcing of both early-stage research and the entire clinical trials phase
- How Etica can capture value from the commercialization of open-source research
Financing Clinical Trials
Etica's approach fundamentally differs from traditional pharmaceutical funding models by distributing the financial risk across the network of token holders rather than relying on single entities making large upfront investments. This unique approach allows Etica to fund the entire clinical trials phase without requiring IP exclusivity.
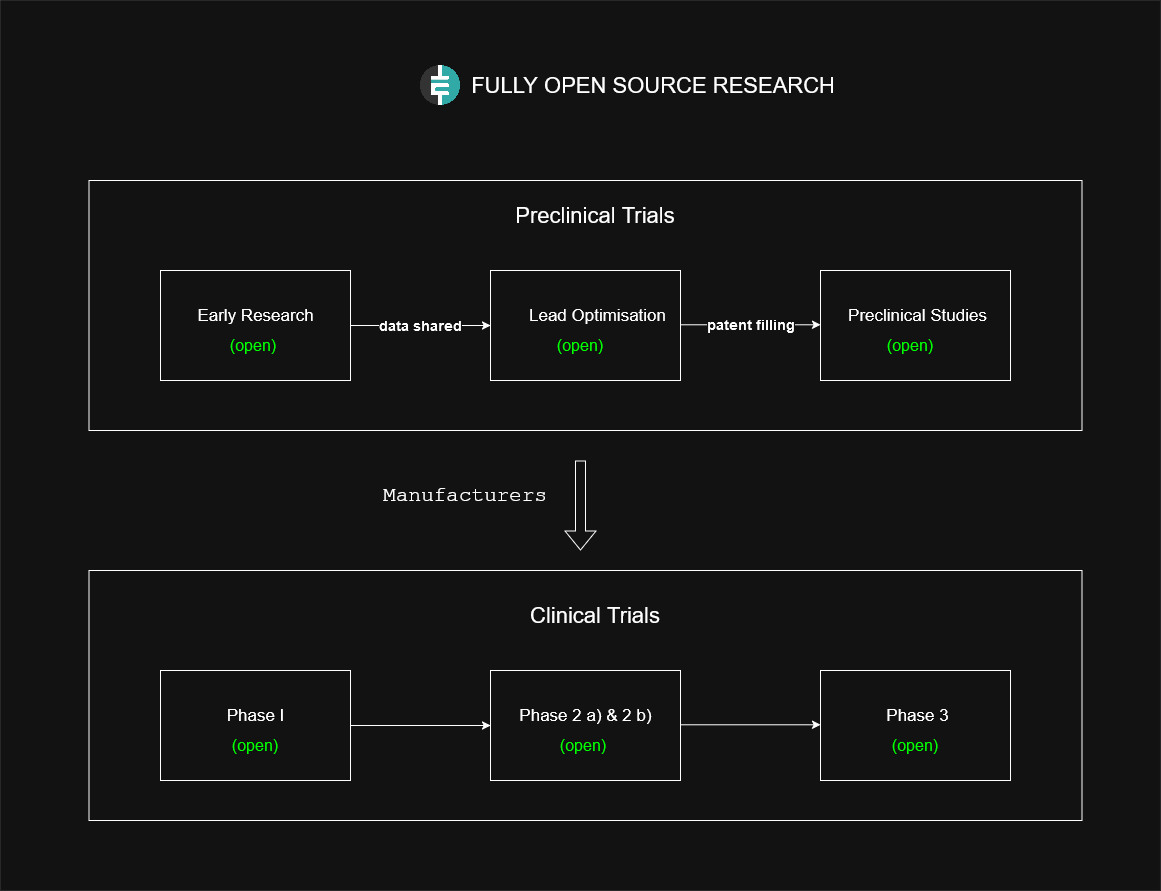
How it works
Etica Protocol offers a revolutionary approach to funding clinical trials through its proposal system, fundamentally transforming how drug development is financed. Clinical trial sponsors, whether they are manufacturers, research institutions, or CROs, can receive immediate funding by submitting detailed proposals for specific phases of clinical trials to the Etica Protocol. Each proposal outlines specific milestones and deliverables, such as patient recruitment targets, safety data collection, or efficacy measurements, which are then evaluated by the Etica community through its native voting system.
The system enables continuous funding throughout the entire clinical trials process. Manufacturers can submit sequential proposals that correspond to different trial phases, from initial Phase 1 safety studies through Phase 2 efficacy measurements to large-scale Phase 3 trial management. When proposals are approved by the community, manufacturers receive immediate ETI rewards from the Period's research rewards, providing real-time compensation for their work rather than requiring them to wait for future drug sales revenue.
The key innovation is that manufacturers don't need to wait years to recover their clinical trial investments through protected drug sales. Instead, the costs of conducting trials are compensated in real-time through ETI rewards as verified progress is made. This immediate compensation removes the need to secure IP rights and market exclusivity, which traditionally serve as the only way to recoup the massive investments required for clinical trials.
Etica creates a virtuous cycle where manufacturers are incentivized to conduct trials efficiently and transparently since their compensation is tied to verified progress rather than future market control. Multiple manufacturers can even collaborate on trials simultaneously, accelerating development while keeping all research open source. This parallel processing approach significantly accelerates the development timeline compared to traditional sequential trials. Rather than having a single entity controlling the entire clinical trial process, multiple research institutions, hospitals, and clinical centers across different regions can participate simultaneously, sharing data and results in real-time through the open-source framework. The result is a sustainable model for funding clinical trials that promotes rapid, collaborative development of new treatments while ensuring they remain accessible to all.
A new circular economy for open-source research
Etica proposes a revolutionary circular economy model for medical research that fundamentally reimagines how value flows in pharmaceutical development. Instead of relying on traditional IP-based exclusivity, the system creates a direct connection between patients, researchers, and manufacturers through blockchain technology. The ecosystem operates in a continuous cycle where patients needs drive research priorities, and researchers conduct their work entirely IP-free. Manufacturers can access this research freely to conduct clinical trials and produce treatments without the burden of IP licensing costs. When patients purchase these competitively priced treatments, they can opt to make OP-R3s payments that flow directly back to the researchers who contributed to the treatment's development.
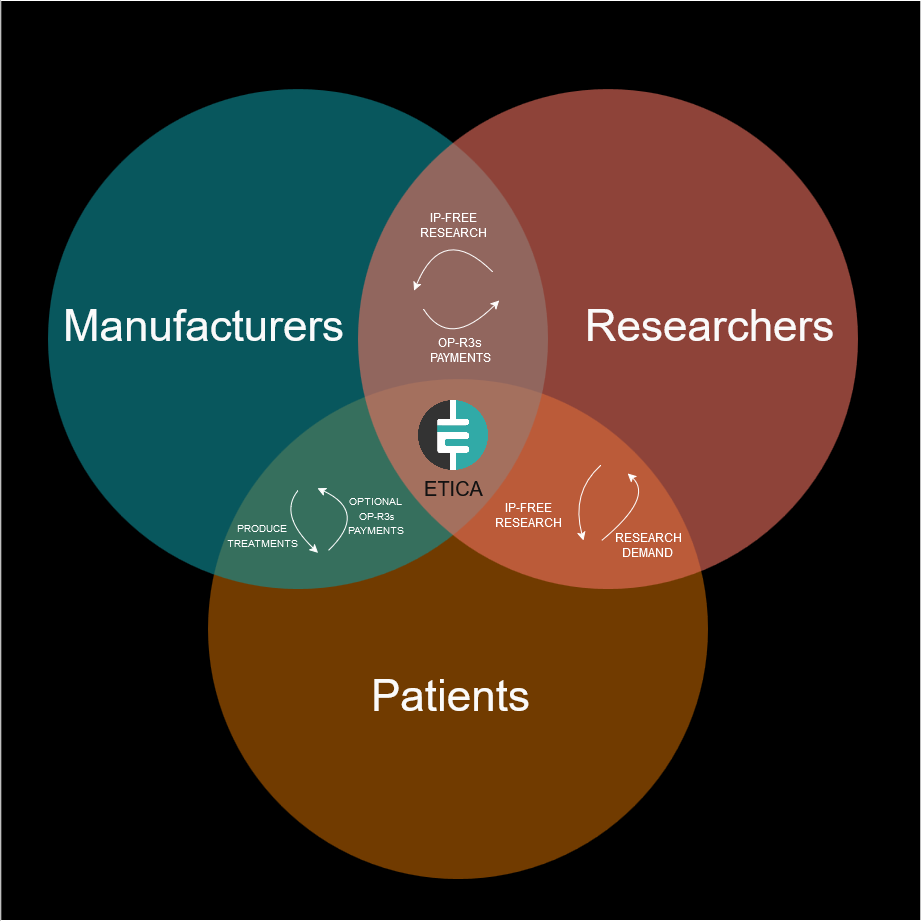
This circular model creates multiple reinforcing benefits. Researchers receive ongoing compensation for their work while keeping their discoveries openly accessible. The absence of IP restrictions enables multiple manufacturers to produce treatments, fostering market competition and driving down prices. Patients gain access to more affordable treatments while having the ability to directly support the research that benefits them. This innovative approach transforms pharmaceutical development from a linear, exclusivity-based model to a circular, collaborative ecosystem. By aligning economic incentives with open science principles, it creates a sustainable framework for advancing medical research while ensuring broader access to its benefits.
The model fundamentally reimagines how value can be captured in pharmaceutical development, shifting from exclusivity-based profits to a direct recognition and reward system that aligns with open science principles while still providing financial incentives for innovation.
Capturing Value from commercialization
Thanks to the OP-R3 layer, Etica can now capture value from the commercialization of open-source research. In this model, when patients purchase treatments, they can optionally contribute a percentage of the purchase price as an OP-R3 payment, which flows directly to the researchers linked to the specific treatment through OP-NFTs. This creates a direct value flow from end-users to researchers without requiring IP patents or artificially inflated prices.
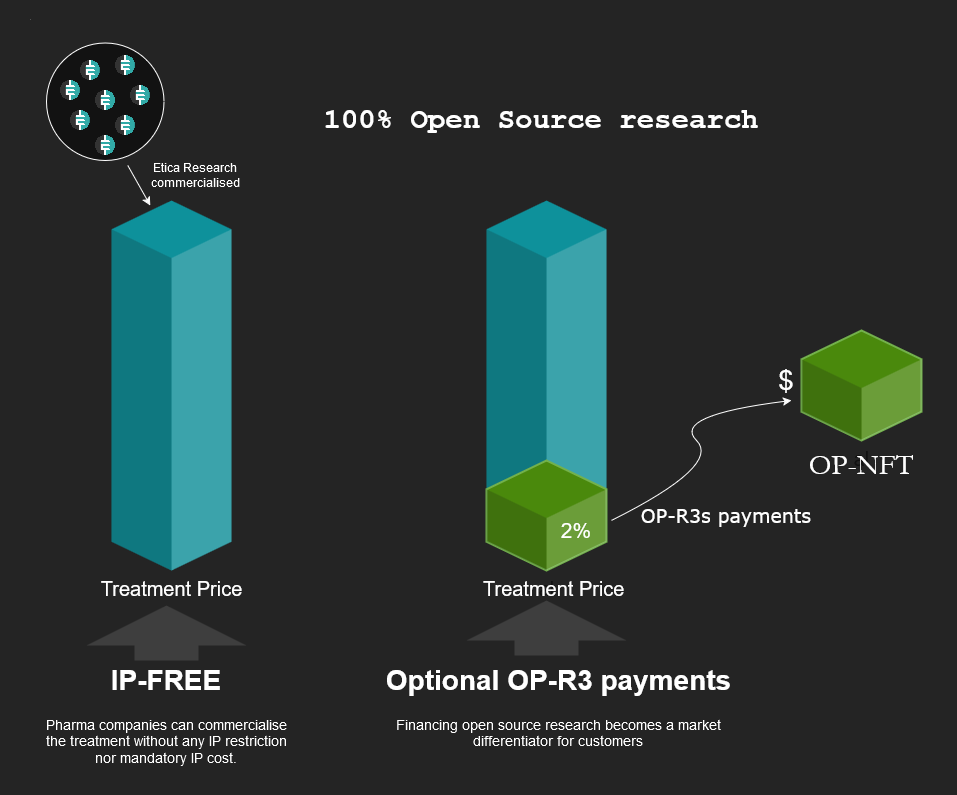
OP-NFTs (Open-Source Non-Fungible Tokens) are financial instruments that represent and track researchers' contributions to open-source medical research. Unlike traditional research funding that relies on patents, OP-NFTs allow researchers to receive ongoing compensation through the OP-R3s layer when their work is referenced or built upon by future research. These tokens function as verifiable proof of research contribution on the blockchain.
When patients purchase treatments developed using open-source research, they can make optional OP-R3s payments that flow back to the researchers based on their contributions tracked through these OP-NFTs. Importantly, OP-NFTs don't restrict access to the research, they simply serve as a mechanism to track contributions and enable fair compensation for researchers over time as their work creates value. For example, if a researcher's early-stage study helps enable the development of a successful treatment, their OP-NFT would allow them to receive a portion of voluntary OP-R3s payments made by patients using that treatment, even years after the initial research was conducted. This creates a sustainable funding model for open-source research without requiring intellectual property restrictions.
Etica Researchers can now get 3 types of funding.
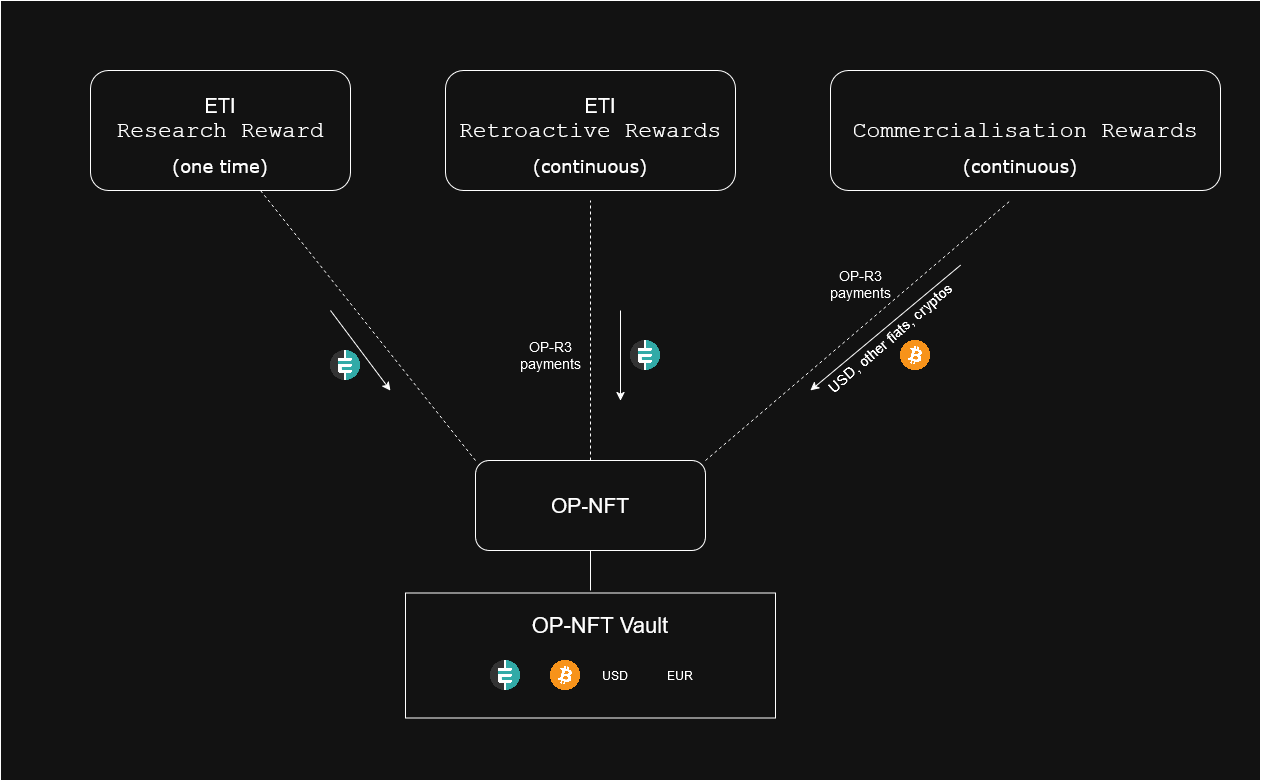
Etica Protocol introduces a three-tiered reward system for open-source medical research that combines immediate funding with long-term compensation. The system creates multiple revenue streams for researchers, ensuring they can be fairly compensated for their work while keeping their research openly accessible.
The first reward stream comes from direct research contributions through Etica Protocol. When researchers publish new research and submit proposals, they can earn a portion of the weekly ETI research rewards. This provides immediate compensation in ETI cryptocurrency as a one-time payment, similar to traditional research grants but with faster distribution and less bureaucracy.
The second reward stream flows through the OP-R3s (Open-Source Retroactive Research Rewards) system within Etica Protocol. When other researchers build upon existing work and reference it in their proposals, the original researchers receive continuous payments in ETI cryptocurrency. This creates an interconnected network where valuable foundational research continues to generate rewards as it enables future discoveries. The more their research enables future developments, the more rewards researchers can earn through this mechanism.
The third reward stream comes from the commercial application of research through patient contributions. When treatments developed from open-source research reach the market, patients can choose to make optional OP-R3s payments in various currencies (USD, EUR, BTC, etc.) that flow back to the researchers whose work contributed to the treatment's development. These payments are tracked through OP-NFTs that document each researcher's contributions, creating a direct link between successful treatments and the underlying research that made them possible. This provides a sustainable source of ongoing funding without requiring patent protection or access restrictions.
Together, these three reward streams create a comprehensive funding model that aligns the interests of researchers, manufacturers, and patients while maintaining the principles of open science and global accessibility.
Automated Research Value Capture
Etica Protocol dramatically simplifies research monetization by creating a streamlined path from research to commercialization. Researchers only need to submit their research proposals to the protocol and attach an OP-NFT to start receiving rewards. The protocol's infrastructure then handles the entire development process, from early research, preclinical studies, and clinical trials up to the point of commercialization. This removes traditional barriers like IP management, licensing negotiations, and funding concerns for clinical trials, allowing researchers to focus purely on their scientific work.
Through Etica's automated monetization system, researchers can focus entirely on innovation rather than commercialization. When researchers submit their work to Etica Protocol, the OP-NFT system automatically handles value capture - no IP management or licensing negotiations required. Their discoveries become freely available to manufacturers worldwide, fostering competition that drives both quality improvements and price reductions.
This enables a fundamental shift in pharmaceutical research: organizations can now specialize purely in discovery and research, earning returns through the OP-R3s layer, while leaving manufacturing and distribution to competitive market forces. For the first time, we see a clear separation between research and production in pharmaceuticals - mirroring the successful model of other industries. The more impactful the research in enabling successful treatments, the greater the automated returns through the OP-R3s system, creating perfect alignment between scientific value and researcher compensation without the overhead of traditional IP-based monetization.
Further Benefits
Better response to global health crises
The Etica Protocol's approach represents a significant advancement in responding to global health crises, addressing key limitations revealed during the COVID-19 pandemic. By enabling decentralized, parallel research and clinical trials, through the removal of IP barriers, the system dramatically accelerates treatment development while ensuring global accessibility. Research teams worldwide can simultaneously conduct trials and share data in real-time, while manufacturers in any country can begin production as soon as safety and efficacy are established.
The model's innovative funding structure, with optional OP-R3s payments, creates a sustainable system that balances global access with research incentives. Low-income countries can immediately produce treatments without IP restrictions or licensing fees, while contributions from high-income countries through OP-R3s payments can sustain research efforts during global health crises. This removes the traditional conflict between rapid response and profit incentives that hampered COVID-19 vaccine distribution.
Just as the COVID Moonshot demonstrated how open science could accelerate antiviral development, Etica Protocol extends this approach by creating a permanent infrastructure for open collaboration. The protocol enables the kind of global coordination needed for pandemic response - immediate sharing of findings, rapid local manufacturing capability, and barrier-free collaboration - while ensuring sustainable funding. This provides the framework for building a robust, globally accessible pipeline of treatments ready for future health crises.

Conclusion
By transforming pharmaceutical development from a patent-based model to a circular economy of open collaboration, the Etica Protocol creates a framework where rapid scientific progress and global accessibility can coexist with sustainable research funding. This system could fundamentally change how we respond to future health crises while ensuring equitable access to life-saving treatments across economic boundaries.